
What is FORMaT?
FORMaT is an adaptive, multi-arm, platform clinical trial designed to find out which combination of medications best treat Mycobacterium abscessus (MABS) chest infections. The treatment of MABS chest infections is complicated and the patient is required to take multiple medications which are often poorly tolerated. The combination of medications used to treat MABS chest infections are also very expensive. However, the evidence for what medication combinations should be used is not well understood. When to start these medications is also not well understood. As such, the FORMaT trial has been designed as an adaptive, multi-arm, platform clinical trial and brings together internationally recognized experts and clinical leaders to solve this treatment challenge.
What is an adaptive, multi-arm, platform clinical trial?
The FORMaT trial has been developed using an adaptive, platform clinical trial design. The trial platform provides a framework that can be used to continuously evaluate different treatment protocols. Initially, the design of the FORMaT trial will evaluate the standard medications prescribed to treat MABS lung disease as these standard medications have not been properly tested for treating this disease (Figure 1). The trial platform can also add new treatment arms into the trial as well as having the ability to remove any treatment arms deemed ineffective. The adaptive part of the trial allows the data to be analysed while the trial is ongoing and these results can be used to change the trial design in real-time. This means that we can quickly identify effective treatment arms and potentially allocate more people to the successful treatment arm/s. Overall, the FORMaT trial is designed to find effective treatment solutions to treat MABS lung disease.
The FORMaT Trial consists of two participant groups:
1- Intervention Group: Require medications to get rid of their MABS lung disease.
2- Observation Group: Have not met the criteria to treat MABS lung disease infection or have chosen not to take the medications.
For those participants who are to be treated, our specially designed FORMaT database will randomise each participant into an intervention arm depending on a number of clinical factors. There are three intervention arms which have a “base” medication combination that is shared between all intervention arms. One intervention arm is a reference arm and the remaining two intervention arms use the same base medication combination used in the control arm along with repurposed medication added to each intervention arm.
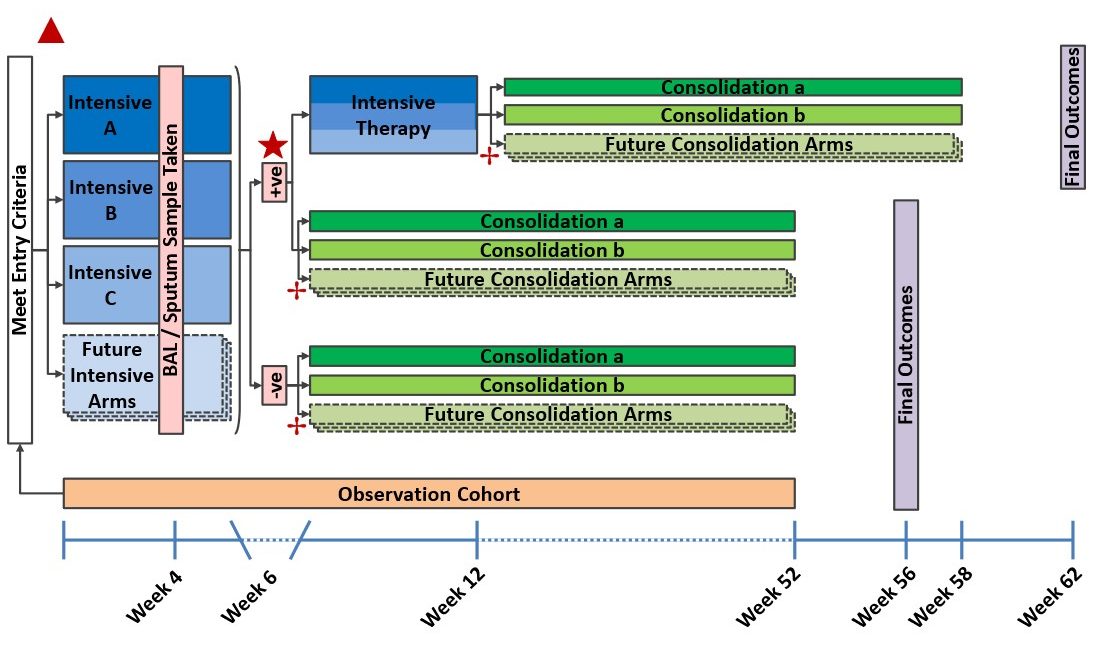
Figure 1: FORMaT trial flow diagram. Eligibility into the intervention program is determined at screening. At randomisation 1 (▲); participants are randomised between the different intensive therapy arms (Intensive A, Intensive B and Intensive C) for a period of 6 weeks. At the end of intensive therapy, it will be determined if participants are still MABS positive, or MABS negative (cleared). Randomisation 2 (★) will ONLY be for participants who are still MABS positive at week six and will allocate participants to either 1) continue intensive therapy or 2) immediately commence consolidation therapy. Randomisation 3 (✢) allocates participants to the consolidation therapy arms either at week 6 or at week 12 depending on MABS clearance at the end of week 6 and randomisation 2 (if relevant). This figure demonstrates the capacity to add future intensive and consolidation arms.
The FORMaT Trial has been registered with the Australian New Zealand Clinical Trials Registry (ANZCTR) and ClinicalTrials.gov:
ANZCTR registration number: ACTRN12618001831279
ClinicalTrials.gov identifier: NCT04310930
Why are we doing the FORMaT trial?
MABS are a group of rapid-growing, multi-drug resistant non-tuberculous mycobacteria (NTM) causing infections in humans, often chest infections. People who have underlying lung conditions such as cystic fibrosis (CF) or bronchiectasis are more likely to get MABS chest infection. The number of people who get MABS chest infections is small, but these infections are becoming more common.
Some MABS chest infections can be cleared without treatment whereas other MABS chest infections can result in MABS lung disease significantly impacting on the person’s quality of life. This is one of the challenges associated with treating MABS chest infections. Currently, there are no guidelines for when to start treating MABS chest infections. The treatment regimens currently used can be administered to the patient for up to two years and are often associated with significant side-effects. The long treatment regimen does not always result in MABS chest infection being cleared from the lungs. These challenges demonstrate that there is an urgent need to find effective treatment regimens to rid the lungs of MABS infection.
Participating in the FORMaT trial
Why take part in this study?
If you have been recently diagnosed with a MABS chest infection you may be eligible to join the study. Speak to your treating doctor if you are interested in joining the trial.
Potential risks of being part of the study
Some people may experience adverse reactions to the medications provided. These risks will be discussed with you before you consent to being part of the study.
Many of the tests which are part of the trial are tests which you would have if you weren’t part of the clinical trial, meaning these tests are considered to be standard practice of care. Some examples of standard practice of care tests would include breathing tests, chest imaging (CT scan or X-ray), blood tests, etc.
Who can participate in the FORMaT trial?
FORMaT trial sites are recruiting participants of all ages and gender who have had at least one positive MABS respiratory sample.
There are two groups of participants in the FORMaT trial:
- The Intervention Group
- Have been diagnosed with MABS lung disease and have decided to start treatment.
- The Observation Group
- Have not been diagnosed with MABS lung disease and do not require treatment, or;
- Have been diagnosed with MABS lung disease but have decided not to start treatment.
Information on Mycobacterium abscessus
What are nontuberculous mycobacterium?
Nontuberculous mycobacterium (NTM) are a group of bacteria naturally present in the environment, such as soil and water. These bacteria can cause significant lung damage in both healthy people and those with underlying lung disease.
There are more than 180 species of NTM. One of these is MABS of which there are three subspecies:
- Mycobacterium abscessus subspecies abscessus (M. a. abscessus)
- Mycobacterium abscessus subspecies massiliense (M. a. massiliense)
- Mycobacterium abscessus subspecies bolletii (M. a. bolletii)
Who gets MABS?
The reasons for why some people are more susceptible to getting MABS chest infections is not well understood. We know that some people are at higher risk of getting a MABS chest infection and developing lung disease as a result of the MABS chest infection. While the overall numbers of people that have MABS chest infections is small, these rates are growing especially in people with an underlying lung conditions such as:
- Cystic fibrosis (CF)
- Bronchiectasis
- Chronic obstructive pulmonary disease (COPD)
- Alpha-1 antitrypsin deficiency
- Primary ciliary dyskinesia (PCD)
How is MABS infection treated?
Treatment for diagnosed MABS lung disease requires a team of skilled and experienced physicians to tailor a treatment protocol to each person. The typical treatment regimen for MABS chest infections involves three or more antibiotics. However, the ideal time to start treatment is not known especially when some people with MABS chest infections can clear the infection without any treatments whereas other people with MABS chest infections will progress to MABS lung disease.
The current medications used for MABS infections are complex and can have significant side-effects for the patient. The medications can cause the patient to become unwell due to either the toxicity of the medications used or due to the medications being poorly tolerated by the patient. The cost of these medications are very expensive, and the costs continue to escalate with medications administered for up to two years.
Caring for the carer
If you are the caregiver of a person with MABS chest infection, your role is vitally important to support the patient on their journey with MABS. MABS chest infections cause significant stress on the patient but also on the caregivers and the family members who also live with the stress of a chronic illness.
Caring for a loved one with a serious illness like MABS lung disease can cause great disruption to your life, especially as you help your loved one with treatments and the challenges of a changed lifestyle. MABS lung disease is a chronic illness, and chronic illness can often cause a feeling of loss of control or feeling that the illness is a huge burden. These feelings are normal and should not be pushed aside. It helps for both you and the patient to know as much as possible about the illness and treatments, so you can make decisions together.
It’s important to address the emotional and physical issues you face, because each patient needs a strong support system, and as the caregiver, so do you, to be a strong support to the patient.
Ethics
All research involving humans is reviewed by an independent body called an Ethics Committee or Institutional Review Board.
The ethical aspects of this research project involving the adult population have been approved by the HREC of The Prince Charles Hospital (HREC approval number: 53810).
The ethical aspects of this research project involving the paediatric population have been approved by the HREC of Children’s Health Queensland Hospital and Health Service (HREC approval number: HREC/19/QCHQ/58696).
This project will be carried out according to the National Statement on Ethical Conduct in Human Research (2007) This statement has been developed to protect the interests of people who agree to participate in human research studies.
Funding bodies






